SCIENTIFIC publications
Title : Understanding the thermodynamic effects of chemically reactive working fluids in the Stirling engine
Authors : Aya Barakat, Silvia Lasala, Philippe Arpentinier, Pascal Tobaly, Jean-Noël Jaubert.
Date of publication : 2024
References :
Energy Conversion and Management: X
Volume 22,
2024,
100576
https://doi.org/10.1016/j.ecmx.2024.100573
Abstract:
The Stirling engine, renowned for its high theoretical efficiency, is capable of partaking an active role in the current energy transition. Given its closed-cycle operation, the choice of the working fluid is pivotal in designing the Stirling engine. Dinitrogen tetroxide N2O4 has been extensively investigated in the past, based on the ideal gas thermodynamic model, as a chemically reactive working fluid for Stirling cycles. This molecule reversibly and rapidly dissociates into nitrogen dioxide NO2 – and recombines into N2O4 – under the influence of thermodynamic transformations throughout the cycle, in accordance with chemical equilibrium. Addressing discrepancies in previous studies, this work aims at assessing a wide range of theoretical chemically reactive gases as working fluids in a Stirling cycle, employing the ideal gas mixture model. The behavior of each reactive fluid is examined throughout the cycle, and the thermodynamic performance is evaluated. Therefore, this work quantifies and analyzes the thermodynamic performance of a chemically reactive Stirling engine. Results indicate a slight increase in the net specific work output with certain reactive fluids, offering a thermal efficiency comparable to that of inert working fluids. In addition, it is emphasized that for chemically reactive working fluids, the isochoric heat exchange within the internal regenerator is incomplete due to chemical reactions, in contrast to the case of inert fluids. To address this, either a supplementary heat source, heat sink, or both are required during the isochoric processes. Furthermore, chemically reactive fluids in the Stirling engine induce irreversibility in the internal regenerator, stemming from heat exchange across a finite temperature difference, penalizing the thermal efficiency of the engine for the majority of reactive fluids studied.
Title : Multiscale modeling of dimerization thermodynamics of formic acid
Authors : Dominika O. Wasik, Silvia Lasala, Olivier Herbinet, Konstantin Samukov, Sofía Calero, Thijs J.H. Vlugt
Date of publication : 2025
Abstract:
Heat pumps, which recycle waste heat, are a promising technology for reducing CO
emissions. Efficiently using low-grade waste heat remains challenging due to the limitations of standard heat exchangers and the need for more effective working fluids. This work introduces a multi-scale methodology that combines force field-based Monte Carlo simulations, quantum mechanics, and equations of state to explore the potential of formic acid as a new reactive fluid in thermodynamic cycles. Formic acid exhibits dimerization behavior, forming cyclic dimers in the gas phase, which can enhance the thermodynamic efficiency of heat recovery systems. The dimerization reaction of formic acid is crucial because it integrates chemical energy into thermodynamic processes, potentially improving the performance of heat pumps and other energy systems. The study implements umbrella sampling in Monte Carlo simulations to compute the thermodynamic properties of HCOOH dimerization, including equilibrium constants, enthalpy, and entropy. Results from two different methods to study dimer formation, namely the dimer counter method and the potential of mean force method, show strong agreement with the enthalpy of dimerization of −60.46 kJ mol−1 and −62.91 kJ mol−1, and entropy of −137.36 J mol−1K−1 and −146.98 J mol−1K−1, respectively. A very good agreement of the Monte Carlo results with Quantum Mechanics and experimental data validates the accuracy of the simulations. For phase equilibrium properties, the Peng–Robinson equation of state, coupled with advanced mixing rules, was applied and compared to Monte Carlo simulations in the Gibbs ensemble. This approach enabled the determination of the Global Phase Equilibrium of the system, vaporization enthalpy, phase composition, vapor and liquid densities of the coexisting phases, and entropy as a function of temperature. The agreement between the thermodynamic model and Monte Carlo simulations confirms the reliability of the methodology in capturing the phase behavior of the system. The findings demonstrate a promising approach for discovering and characterizing new reactive fluids, contributing to more efficient and sustainable energy technologies.
Authors : Konstantin Samukov, David Vega-Maza, Eric W. Lemmon, Vladimir Diky & Silvia Lasala
Date of publication : 2025
Abstract:
A new thermodynamic model is presented, capable of accurately representing the vapour–liquid equilibrium pressures and densities, and liquid phase densities and enthalpies of dissociating dinitrogen tetroxide (N2O4 ⇄ 2NO2). The model is based on the Peng-Robinson equation of state coupled with advanced mixing rules. The -required but non-measurable- critical coordinates of the pure components forming the reactive mixtures are optimized, within a variability range defined in a previous study, to fit experimental vapour–liquid equilibrium data. The optimized parameters are then validated by comparing calculated thermodynamic properties with available experimental data in the subcritical region. The negligible impact of the higher temperature reaction 2NO2 ⇄ 2NO + O2, within the vapour–liquid equilibrium region where the optimisation is performed, is also proven. The resulting model is finally compared with the currently most accurate available equation of state, showing comparable results when considered both the scatter in available experimental data and the relative simplicity of the proposed equation of state. In particular, the proposed model demonstrates the satisfactory capability of a cubic equation of state to accurately reproduce both saturation pressures and saturation densities without requiring volume translation.
Title : Towards an improved thermodynamic modelling and enhanced efficiency of energy conversion systems
Authors : Silvia Lasala
Date of publication : 2024
Silvia Lasala’s habilitation report
Title : Application of thermodynamics at different scales to describe the behaviour of fast reacting binary mixtures in vapour-liquid equilibrium
Authors : Silvia Lasala, Konstantin Samukov, H. Mert Polat, V´eronique Lachet, Olivier Herbinet, Romain Privat, Jean-Noël Jaubert, Othonas A. Moultos, Kevin De Ras, Thijs J. H. Vlugt.
Date of publication : 2024
References :
Chemical Engineering Journal,
Volume 483,
2024,
148961
https://doi.org/10.1016/j.cej.2024.148961
Abstract:
The use of reactive working fluids in thermodynamic cycles is currently being considered as an alternative to inert working fluids, because of the preliminarily attested higher energy-efficiency potential. The current needs to simulate their use in thermodynamic cycles, which may operate in liquid, vapour or vapour-liquid state, are an accurate real-fluid equation of state and ideal gas thermochemical properties of each molecule constituting the mixture, to calculate the equilibrium constant. To this end, the appeal to a multi-scale theoretical methodology is paramount and its definition represents the objective of the present work. This methodology is applied and validated on the system N2O4 ⇌ 2NO2. Firstly, the equations solved for simultaneous two-phase and reaction equilibrium are presented. Secondly, ideal gas thermochemical properties of N2O4 and NO2 are computed at atomic scale by quantum mechanics simulations. Then, to apply the selected cubic equation of state, pure-component properties of the species forming the reactive mixture (critical point coordinates and acentric factor) are required as input. However, these properties are not measurable, since NO2 and N2O4 do not exist in nature as pure components. To get around this difficulty, the methodology relies on molecular Monte Carlo simulations of the pure N2O4 and NO2, as well as on the reactive N2O4 ⇌ 2NO2, enabling the determination of
those missing pure-component properties and thus the calculation, on a macroscopic scale, of the reactive mixture properties. Finally, the comparison of calculated mixture properties with available experimental data leads to validate the accuracy of the proposed methodology.
Title : The original and impactful exploitation of chemical energy in heat pumps
Authors : Aya Barakat, Silvia Lasala, Philippe Arpentinier, Jean-Noël Jaubert
Date of publication : 2022
References :
Chemical Engineering Journal Advances,
Volume 12,
2022,
100400,
https://doi.org/10.1016/j.ceja.2022.100400
Abstract: In light of reducing carbon emissions and the tension on non-renewable energy sources, heat pumps are being investigated as an alternative to fossil-fuel-dependent energy converters in heat demanding applications. On the basis of the impactful results the authors obtained in previous research around the potential of using reactive fluids–instead of inert ones–as novel working fluids in power cycles, the study has been extended to heat pump systems. The thermodynamic analysis of heat pumps operating with reactive gases as working fluids is thus the objective of the present work. More specifically, the analysis is based on the use of instantaneously equilibrated fictive gaseous reactions, with the aim to thoroughly assess the impact of different stoichiometries and thermochemical characteristics of chemical reactions. This studied heat pump is based on the reverse Brayton cycle. Furthermore, the behavior of the fluid in each unit operation of the cycle is investigated and all the results are compared to those of a heat pump utilizing comparable inert fluids; that is to preliminarily quantify the potential gains in performance. For the considered spectrum of reactive fluids, operating conditions, and reaction stoichiometries, the corresponding results show a range of potential reactive fluids that can be utilized in heat pumps and reveal an increase of more than 200% in the system’s coefficient of performance compared to inert-fluid heat pumps.
Title : Thermo-chemical engines: Unexploited high-potential energy converters
Authors : Silvia Lasala, Romain Privat, Olivier Herbinet, Philippe Arpentinier, Davide Bonalumi, Jean-Noël Jaubert
Date of publication : 2021
References :
Energy Conversion and Management,
Volume 229,
113685,
https://doi.org/10.1016/j.enconman.2020.113685.
Abstract: Thermal engines, particularly closed power cycles, are currently a focus of many studies mainly because they represent the only way to exploit renewable thermal energy. To increase the exploitation of available thermal sources, this work investigates the higher potential offered by a complementary technology based on the use of reactive working fluids instead of inert fluids: the here-called “thermo-chemical” engine. Such a power cycle enables the simultaneous conversion of thermal and chemical energy into work. Based on a theoretical approach, this paper explores engine performance considering different stoichiometries and thermodynamic characteristics of reactive fluids and different operating conditions. It is shown that the use of specific equilibrated reactions occurring in the gaseous phase might lead to extremely powerful and highly efficient energy conversion systems in the whole current domain of the application of power cycles. Moreover, it is demonstrated that, unlike classical thermal machines, a thermo-chemical engine allows efficient and powerful exploitation of low-temperature heat sources and high-temperature cold sinks, which in general, characterize renewable thermal energy.
disclosure publicationS
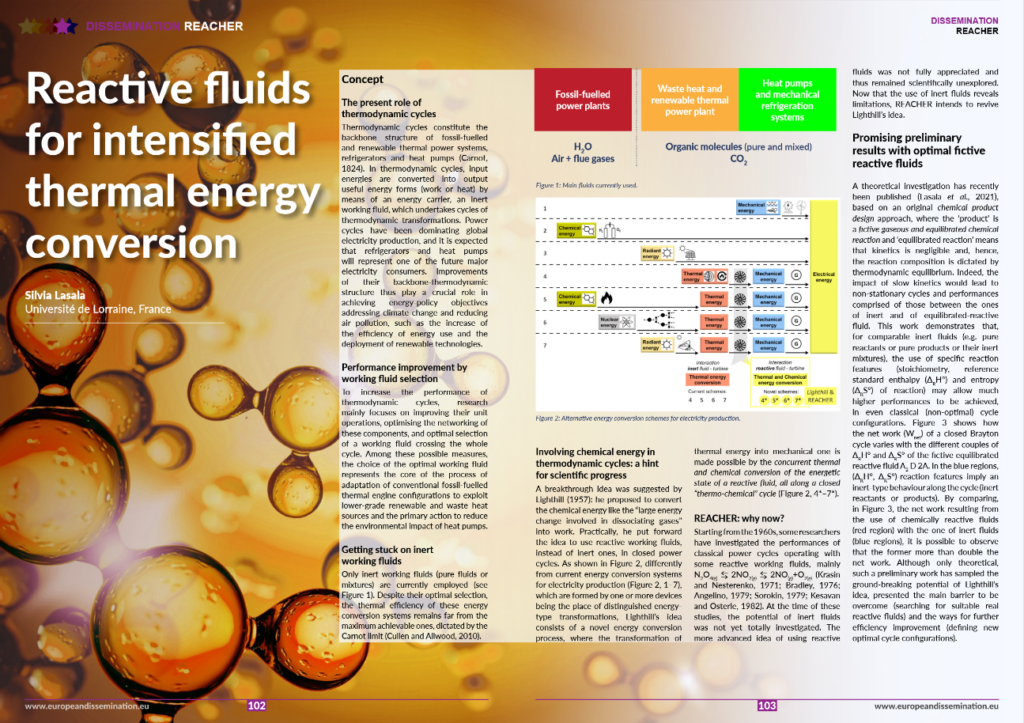
The Project Repository Journal devoted an article to the Reacher project.
Find the explanation of the project on pages 102 to 105.

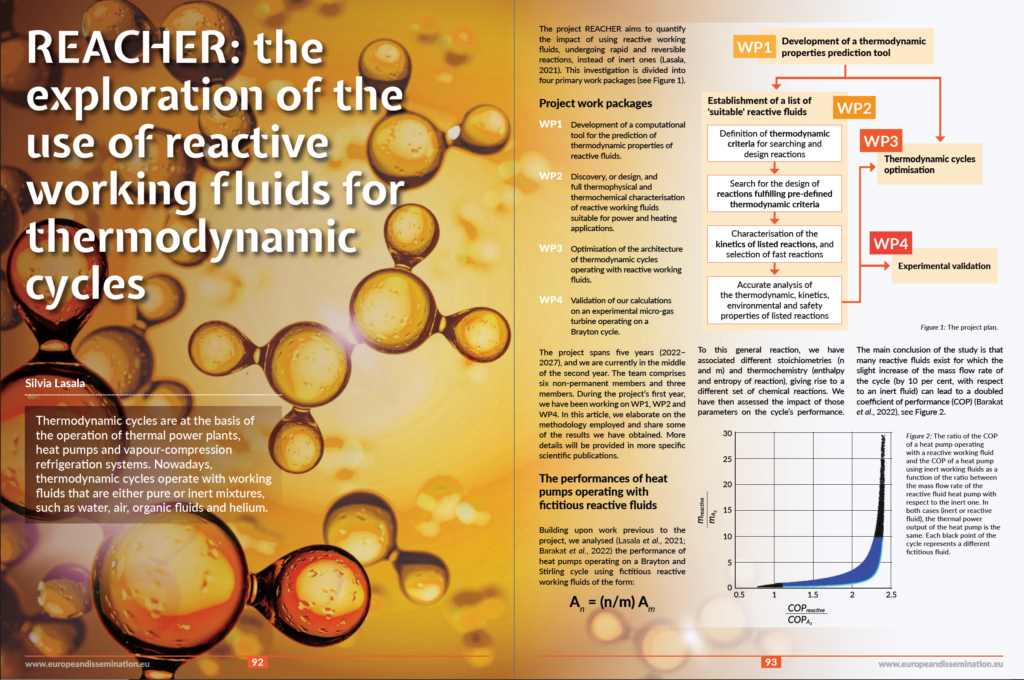

The Project Repository Journal devoted an article to the Reacher project.
Find the explanation of the project on pages 92 to 95.